CCM® Therapy
CCM® therapy is an innovative intracardiac device-based therapy has been utilized in the treatment of patients with chronic heart failure, left ventricular ejection fraction (LVEF) ≥25 and ≤45%, who remain symptomatic despite optimal medical therapy (OMT). CCM® therapy patients experience a higher quality of life by reducing CHF symptoms such as overwhelming shortness of breath and fatigue.
Who is indicated For?
Patients with the following indications are candidates for CCM® Therapy.
Ejection Fraction 25-45%
Class III Heart Failure
Guideline Directed Medical Therapy
Not indicated for Cardiac Resynchronization Therapy
Clinical Evidence
Clinical trials have demonstrated statistically significant and clinically meaningful improvements in the FIX-HF-5C randomized trial.
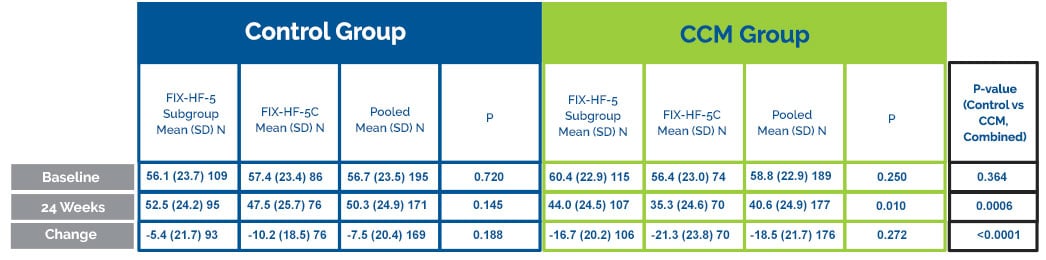
Improvment in MLWHFQ
CCM® subjects improved 11.7 points in the MLWHFQ score compared to the control group.
MLWHFQ
P-values from the two-sample t-test of the mean differences. Results from the repeated measures mixed model on the change form baseline at 24 weeks including all available data in the model yield a least squares mean difference between treatment and control in the pooled cohort of -10.9 (p<0.0001).
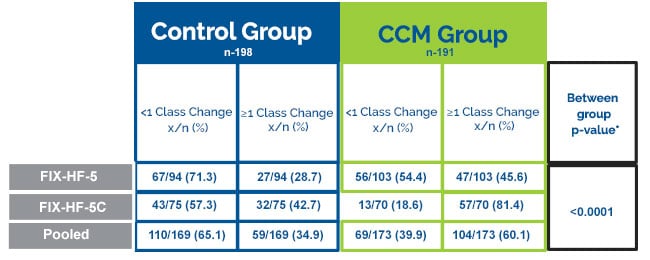
NYHA Class Improvement
CCM® subjects improved their NYHA heart failure status by >1 class 81% of the time compared to 42% in the control group.
NYHA Class Improvement
Numbers and percentages of patients whose NYHA increased by 1 or more class at 24 weeks. Comparison of results from the FIX-HF-5 and FIX-HF-5C studies and in an analysis where data are pooled from both studies
*P-value from Cochran-Mantel-Haenszel test, stratified by baseline etiology
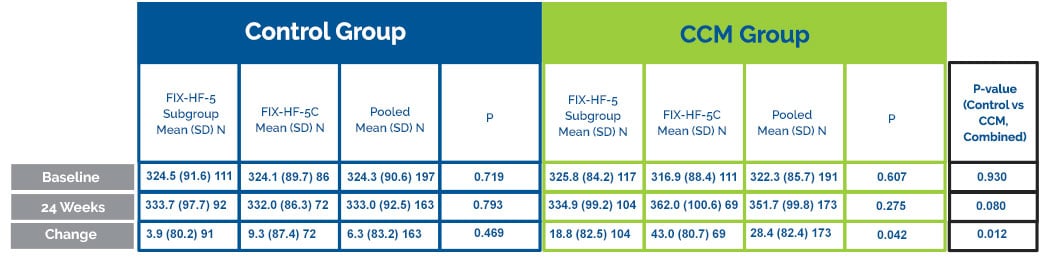
Six Minute Walk Improvement
CCM® subjects walked on average 33.7 meters further than the control group during the 6-minute hall walk test
Frequently Asked Questions
What are the complication rates (compared to typical ICD or PPM procedures)?
Our clinical trials have shown 30 day post-implantation complication rates similar to that for dual chamber pacemakers.
What percentage of patients get better?
81.4% of HF patients treated with CCM saw at least a 1 NYHA class improvement in the FIX-HF-5C trial. Trials consistently report positive results using standard measures of functional capacity (6MHWD) and Quality of Life (MLWHFQ)
What are the key Clinical Trials backing this?
FIX-HF-5C/FIX-HF-5C2 were the pivotal trials we submitted for FDA approval of the 3-lead and 2-lead Optimizer systems respectively. These studies are posted to our website @ https://impulse-dynamics.com/providers/clinical-trials/
Which endpoints in which trials led to FDA approval?
FIX-HF-5C showed CCM meeting endpoints for peak vo2, QoL and NYHA Class improvement which led to the FDA approving the device
What kind of improvement in EF can a patient expect?
We have not studied changes in EF as a predefined endpoint, and as such cannot make claims regarding EF improvement. But know that three studies (Imai: dogs; Yu: humans; CCM-Reg) all showed EF improvement, typically 4-5% points in absolute terms.
You just mentioned a 10 point improvement in QoL. What does that really mean?
Actually, as measured by the MLWHFQ, patients experienced an 11.7 point improvement in QoL score, which is associated with an improved sense of well being and an improvement in the ability to engage in the activities of daily life.
What data do you have to support the claim that people will be kept out of hospital if they have CCM?
We don’t have any clinical trails focusing on the hospitalization reduction endpoint to determine if CCM leads to such positive outcomes. So, we cannot say or claim that CCM therapy will reduce hospitalization. Our long-term Registry study does show significant drops in hospitalization rates;Specifically, CCM use was associated with a 71% reduction in the rate of cardiovascular and heart failure hospitalizations in the CCM-REG25-45 cohort, an approximately 80% reduction in the CCM-REG35-45 cohort and a roughly 65% reduction in the CCM-REG25-34 cohort when compared with the year prior to CCM therapy.
What is the difference betweeen peak VO2 and ventilatory anaerobic threshold?
Peak V02 is the highest level of oxygen used as you exercise at increasing intensity; The ventilatory anaerobic threshold represents, to an extent, the maximal amount of work an individual can sustain for a prolonged period of time.
Why did you go from a 3 lead to a 2 lead?
Our 2 lead system was shown to be just as effective in delivering therapy, and by reducing hardware the expectation is that complication rates will be reduced.
Why did the FDA approve the 2 lead device?
It met the endpoints of the trial in being equally effective and safe in delivering CCM therapy.
Mechanism of action
How does CCM®work?
The animation shows how the genes of a patient with heart failulre can become down-regulated, while others become abnormally up-regulated. CCM Therapy has been associated with reversion of these key gene expression back towards normal profiles, which collectively improves myocardial and LV functions.
Refer to Instructions for Use for additional information.
ABOUT Impulse Dynamics
Who is Impulse Dynamics?
Impulse Dynamics is a medical device company determined to transform the treatment of chronic heart failure. We are professionally directed by an expert team of world-renowned scientists, engineers, physicians, and business executives. Our research and commercial operations across the globe include corporate headquarters in the United States (Marlton, NJ), the Netherlands Antilles, Germany, and Hong Kong. We conduct research in major universities and hospitals worldwide where we are currently engaged in clinical trials both in the United States and Europe. Our company was incorporated on May 11, 1998 as a subsidiary of the Hobart Group under the guidance our founder, Professor Shlomo Ben-Haim. With qualifications in medicine, biophysics, nuclearphysics, mathematics, and philosophy, Ben-Haim is a luminary figure both in medicine and across the life sciences industry. He is a relentlessly prolific researcher and inventor with more than 550 patents to his name. Among his many notable achievements is the foundational navigated cardiac catheterization technology that enables Biosense-Webster’s CARTO EP navigation system.
About CCM® Therapy
Impulse Dynamics offers cardiac contractility modulation therapy to treat adults with moderate-to-severe chronic heart failure. CCM® delivers non-excitatory electrical signals to the right side of the intraventricular septum during the absolute refractory period of the ventricular contraction, and does not trigger a new action potential. CCM® signals are delivered by Impulse Dynamics’ Optimizer system (i.e. Optimizer Smart), which is an implantable pulse generator. This device is implanted in a minimally-invasive procedure under local anesthesia, typically in the right pectoral region. CCM® has been evaluated in patients with heart failure with reduced ejection fraction (HFrEF) In contrast to a pacemaker or a defibrillator, the system is designed to modulate the strength of contraction of the heart muscle rather than the rhythm. CCM® therapy is delivered at regular intervals throughout the day.

Insurance Coverage
We have pathways to payment with Medicare and commercial payors in multiple care settings – inpatient, outpatient and surgery centers. Additionally, we have a range of resources available for facilities, practitioners and patients related to obtaining access to Optimizer®.
Impulse Dynamics has committed ourselves to maximizing availability of Cardiac Contractility Modulation to patients who would benefit from it. To learn more, please contact your local Impulse Dynamics Territory Manager or email reimbursement@impulse-dynamics.com
Provider Testimonials
What Providers are saying about Optimzer® Smart Therapy
“I’ve been amazed at how virtually every single one of my patients has seen a transformation in their life, having received this therapy.”
“The FDA granted breakthrough designation to this device and the reason they did that is because this is the first device-based therapy in this population that can reduce symptoms when medications alone are not enough.”
“I’ve had patients that were wheelchair bound that were able to walk again.”
“I think if you’re feeling that you have run out of options for the treatment of your heart failure and you haven’t had a conversation with a cardiologist or an electrophysiologist about the role of CCM®, then I think that there are still potential options for you, and you really aren’t at the end of the road.”

© 2024 Impulse Dynamics Terms of Use | Data Protection | Vulnerability Disclosure | Terms and Conditions